


AND CONTRAINDICATIONS
In patients exposed to tuberculosis. The risk-benefit ratio of the drug should be evaluated before using Olokizumab in such patients.
In patients with a history of diverticulitis or intestinal perforations and other risk factors for intestinal perforation.
In patients with hepatic impairment or hepatic failure.
Active infectious diseases (including tuberculosis).
Children under 18 years of age.
Hereditary fructose intolerance (the drug contains sorbitol).
Breastfeeding..








- Preclinical studies
- Within the framework of Phase I and II clinical trials
- Three international Phase III clinical trials involving 2,444 patients over 18 years of age with moderate to severe rheumatoid arthritis (CREDO 1, 2, 3)
- An open-label long-term study of patients (CREDO 4) who completed one of the Phase III clinical trials

(12 weeks)
(12, 24 weeks)
DAS28<3,2
CDAI≤2,8
HAQ-DI
DAS28<3,2
CDAI≤2,8
HAQ-DI
DAS28<3,2
CDAI≤2,8
HAQ-DI

- Long-term (up to 82 weeks of observation) retention of patients on therapy and maintenance of the achieved response according to the main effectiveness indicators
- No new safety signals compared to other IL-6 drugs
- Low immunogenicity of olokizumab throughout the observation period





3 countries globally (Belarus, Bulgaria, Russian Federation)













18 countries globally (in US, European Union (EU),United Kingdom (UK), Russian Federation, Asia, Latin America)













11 countries globally (in US, EU, Russian Federation, Asia, Latin America, United Kingdom, United States)




When switching from placebo to olokizumab therapy at week 16 of the study, patients achieved a clinically meaningful response



18 countries globally (Argentina, Belarus, Brazil, Bulgaria, Colombia, Czechia, Estonia, Germany, Hungary, Korea, Republic of, Latvia, Lithuania, Mexico, Poland, Russian Federation, Taiwan, United Kingdom, United States).





N = 1 582
N = 4543








safety profile is consistent with the IL-6 inhibitor class






- ACR 20,50,70
- DAS28-CRP(ESR)
- SDAI
- CDAI
- ADL: Activities of daily living Boolean-1, Boolean remission with PGA ≤ 1, TJC ≤ 1, JC ≤ 1, and CRP ≤ 1
- Boolean-2: Boolean remission with PGA ≤ 2, TJC ≤ 1, SJC ≤ 1, and CRP ≤ 1
- Functional impairment according to the HAQ-DI index (from 0 to 3 points)

PGA and EGA approximating on a scale 1-10 (on a VAS)

satisfactory effect - CDAI reduction by 7 points,
good effect - CDAI reduction by 15 points


- functional impairment according to the HAQ-DI index (from 0 to 3 points)
- overall assessment of disease activity by the patient (PAAD; Patient's Global Assessment of Disease Activity, PtGA) (from 0 to 100 mm) using a visual analogue scale (VAS)
- total pain score according to VAS (from 0 to 100 mm)
- fatigue on the FACIT Fatigue (Functional Assessment of Chronic Illness Therapy) scale (0 to 52 points)
- quality of life according to the EQ-5D questionnaire (European Quality of Life-Five-Dimension Questionnaire)
- physical and mental components of the SF-36, including physical functioning, impact of physical condition on daily role functioning (work, daily duties), pain intensity, general health, vitality, social functioning, affective role functioning, and mental health
Do not heat up the product.
- it has been out of the refrigerator for more than 4 hours,
- it contains the wrong medication name,
- the shelf life indicated on the package has expired,
- the vial or pre-filled syringe is cracked, damaged or leaking,
- the solution is cloudy, discolored, or contains flakes or particles.
The drug in a pre-filled syringe is ready for use.
Prepare the drug in a vial for use following the instructions
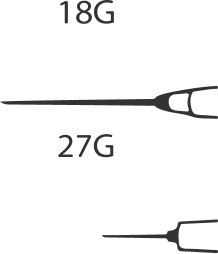
Prepare a 1-2 mL disposable hypodermic syringe, two disposable sterile needles (it is recommended to use an 18G needle to draw the drug from the vial and a 27G needle to perform a subcutaneous injection), 2 sterile alcohol wipes.
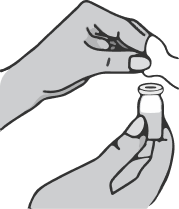
Take the syringe in your right hand, remove the cap from the needle and insert the needle vertically into the center of the vial stopper so that the tip of the needle appears on the other side of the stopper.
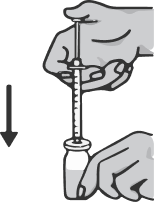
Holding the syringe with your right hand, take the vial with your left hand and turn it upside down so that all the liquid collects over the stopper. Pulling the plunger of the syringe down, draw the entire contents of the vial into the syringe.
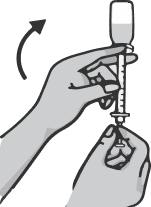
Pull the needle out of the vial and place the cap back on it. Without removing the cap, replace it with a hypodermic needle. Dispose of the used drawing needle into the sharps container
Do not perform the injection with the drawing needle as this may cause pain and damage at the injection site.
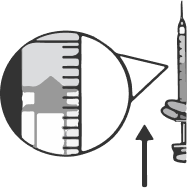
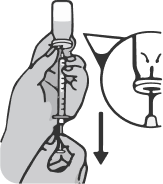
Place the syringe on the carton so that the needle stays on top of the package and does not touch other surfaces.
Select an injection site
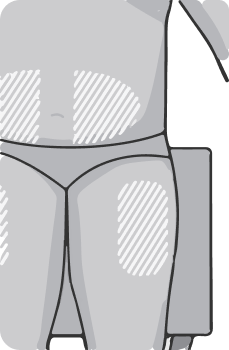
Select an injection site on the upper thighs or abdomen, at least 5 cm from the navel. If the injection is performed by a healthcare professional, other sites for subcutaneous injections may be selected. The drug should not be injected into moles, scars, lesions or indurations, areas of redness, skin hypersensitivity reactions.
It is recommended to change the injection site regularly. The new injection site should be at least 2.5 cm from the previous one. You can alternate injection sites between the thighs and abdomen.
Perform the injection
Clean the skin of the selected injection site with a new sterile alcohol wipe. Allow the injection site to dry and do not touch it prior to injection.
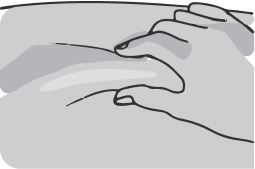
Gently squeeze the skin around the injection site with your non-dominant hand (for example, if you are right-handed, use your left hand) and hold it firmly.
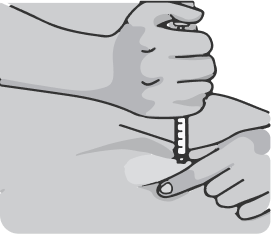
Hold the syringe in your dominant hand over the raised area of skin at a 90 degree angle. Insert the needle into the skin with a quick, smooth thrusting motion. If there is a low amount of subcutaneous fat on the abdomen, a 45-degree angle may be used..
Do not move the needle relative to the tissue and push the plunger down slowly until the entire volume of the drug from the syringe is injected under the skin. The plunger should reach the bottom of the syringe. Wait a few seconds before removing the needle. Pull the needle out of the skin at the same angle at which it was inserted. There may be slight bleeding at the injection site. If necessary, place a sterile wipe on the injection site.
Dispose of used syringes in the sharps container immediately after injection. When the container is full, close it carefully and tightly and discard it in a trash can.
Always use a new syringe, do not reuse syringes.
Genovese MC, Fleischmann R, Furst D, et al .Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study .Annals of the Rheumatic Diseases. 2014;73:1607-1615.DOI: 10.1136/annrheumdis-2013-204760
Tsutomu Takeuchi, Yoshiya Tanaka, Hisashi Yamanaka, Kanzo Amano, Ryuji Nagamine, Won Park, Kazuko Shiozawa, Michishi Tsukano, James Cheng-Chung Wei, Jing Shao, Osamu Togo & Hideki Mashimo . Efficacy and safety of olokizumab in Asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: Results from a randomized phase II trial Modern Rheumatology. 2016, 26:1, 15-23. DOI: 10.3109/14397595.2015.1074648
Bure I. Mikhaylenko D, Kuznetsova E., Alekseeva E, Bondareva K., Kalinkin A., Lukashev A., Tarasov V., Zamyatnin A Jr., Nemtsova M.Analysis of miRNA Expression in Patients with Rheumatoid Arthritis during Olokizumab Treatment.J. Pers. Med. 2020, 10(4), 205;DOI: org/10.3390/jpm10040205
Genovese MC, Durez P, Fleischmann R, Tanaka Y, Furst D, Yamanaka H, Korneva E, Vasyutin I, Takeuchi T. Long-term safety and efficacy of olokizumab in patients with rheumatoid arthritis and inadequate response to tumor necrosis factor inhibitor therapy in phase II studiesEur J Rheumatol 2021 Jul;8(3):120-129. "DOI: 10.5152/eurjrheum.2021.19207PMID: 34101570PMCID: PMC9770405“
Nasonov E, Fatenejad F., Feist E., Ivanova M, Korneva E , Krechikova D. , Maslyanskiy A., Samsonov M, Stoilov R., Zonova E, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomized controlled phase III study Ann Rheum Dis . 2021;0:1–11. DOI: org/10.1136/annrheumdis-2021-219876
Buryachkovskaya L, Nikita Lomakin N, Melkumyants A, Docenko J, Serebruany V..Impact of olokizumab on platelets, leukocytes and erythrocytes during mild COVID-19 Rev. Cardiovasc. Med. 2021 vol. 22(3), 549-551. DOI: 10.31083/j.rcm2203065
Feist e, Fatenejad s, Grishin s, Elena Korneva, Michael Luggen, Evgeniy Nasonov, Mikhail Samsonov, Josef S Smolen, Roy M Fleischmann. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis 2022;81:1661–1668.DOI: org/10.1136/ard-2022-222630
Josef S. Smolen, Eugen Feist, Saeed Fatenejad, Sergey A. Grishin, Elena V. Korneva, Evgeniy L. Nasonov, Mikhail Y. Samsonov, and Roy M. Fleischmann. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med . 2022; 387:715-726. DOI: 10.1056/NEJMoa2201302
Mahmoud MA.Olokizumab’s effectiveness and safety in patients with rheumatoid arthritis: A systematic review and meta-analysis of randomized controlled trials.Journal of Clinical Densitometry: Assessment & Management of Musculoskeletal Health, vol. &2022 Elsevier Inc.DOI: org/10.1016/j.jocd.2022.12.003
Mikhaylenko, D.S., Kuznetsova, E.B., Musatova, V.V., Bure, I.V., Deryagina, T.A., Alekseeva, E.A., Tarasov, V.V., Zamyatnin, A.A., Jr., Nemtsova, M.V.Genetic and Clinical Factors Associated with Olokizumab Treatment in Russian Patients with Rheumatoid Arthritis.J.Pers. Med. 2022, 12, 641. DOI: rg/10.3390/jpm12040641
Eugen Feist and Evgeny Nasonov.Interleukin 6 Inhibition in Rheumatoid Arthritis: Highlight on Olokizumab.touchREVIEWS in RMD.2023;2(1).Online published
Young Ho Lee, Gwan Gyu Song.Comparison of the efficacy and safety of tocilizumab, sarilumab, and olokizumab in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials.Zeitschrift für Rheumatologie.2023 Published online.DOI: org/10.1007/s00393-022-01315-0
Young Ho Lee · Gwan Gyu Song.Comparison of the efficacy and safety of olokizumab at different dosages in patients with active rheumatoid arthritis: a network meta-analysis of randomized controlled trials.Zeitschrift für Rheumatologie.Published online 2 June, 2023.DOI: org/10.1007/s00393-023-01367-w
Please note that by sending information to safety@rpharm.ru, you consent to the processing of your personal data by R-Pharm JSC. Weguarantee that any personal data received will be processed solely in accordance with R-Pharm JSC's policy on personal data processing. The text of the policy is available at www. You may withdraw your consent to the processing of personal data at any time by sending an application to R-Pharm JSC or by e-mail to safety@rpharm.ru.
- GBD 2021 Rheumatoid Arthritis Collaborators. Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: a systematic analysis of the Global Burden of Disease Study 2021. Lancet Rheumatol 2023; 5: e594–610.
- United Nations, Department of Economic and Social Affairs, Population Division (2022). World Population Prospects 2022, Online Edition.
- Virgin Islands include British Virgin Islands and United States Virgin Islands
- Instructions for the medical use of the drug Artlegia, registration certificate LP- 006218 dated 21.05.2020, updated on 19.02.2024.
- Hunter, C., Jones, S. IL-6 as a keystone cytokine in health and disease. Nat Immunol 16, 448-457 (2015). https://doi.org/10.1038/ni.3153 (Hunter, C., Jones, S. IL-6 as a keystone cytokine in norm and disease. Nat Immunol. May 2015;16(5):448-57. doi: 10.1038/ni.3153. Printed in: Nat Immunol. Oct 18, 2017;18(11): 1271. PMID: 25898198).
- Kaur S, Bansal Y, Kumar R, Bansal G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg Med Chem. 2020 Mar 1;28(5):115327. doi: 10.1016/j.bmc.2020.115327. Epub 2020 Jan 20. PMID: 31992476. https://pubmed.ncbi.nlm.nih.gov/... (Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL-6: structure, pathophysiologic role and inhibitors. Bioorg Med Chem. March 1, 2020; 28 (5): 115327. doi: 10.1016/j.bmc.2020.115327. doi: 10.1016/j.bmc.2020.115327. Epub, January 20, 2020. PMID: 31992476. https://pubmed.ncbi.nlm.nih.gov/...
- Shaw, S. et al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 6, 774-782 (2014) https://www.tandfonline.com/doi... (Shaw, S. et al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 6, 774-782 (2014) https://www.tandfonline.com/doi/...
- Instruction for medical use of the drug Artlegia, registration certificate LP- 006218 from 21.05.2020, update from 19.02.2024.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Van der Heijde D, Daikh DI, Betteridge N,Burmester GR, Hassett AL, Matteson EL, et al.Common language description of the termrheumatic and musculoskeletal diseases (RMDs)for use in communication with the lay public,healthcare providers and other stakeholdersendorsed by the European League AgainstRheumatism (EULAR) and the American Collegeof Rheumatology (ACR). Ann Rheum Dis.2018;77:829-32.2Obesity have been reported to be 18.6%, 6.0%,9.9%, and 4.4%, respectively. (Smolen – Verifyexact source)Impact of gender on the response and toleranceto abatacept in patients with rheumatoid arthritis:results from the ‘ORA’ registry, RMD Open. 20173 WHO Rehabilitation Need Estimator (2019);Inthe tab for the European region, it showsthatRMDs are responsible for at least 50% ofYLDsuntil the age range gets into the mid-70s. Inthe‘location tab’ it shows that in Europe 26.9K ofapproximately 40K people in need ofrehabilitationin 2019 hadRMDs. https://vizhub.healthdata.org/... .
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Clinical Guidelines 2021 Rheumatoid Arthritis. www.cr.minzdrav.gov.ru/... Accessed March 18, 2022.
- Nasonov EL, Karateev DE. Does Russia need a treat-to-target initiative? Rheumatology (Oxford) 2015;54(3):381–382. doi: 10.1093/rheumatology/keu156. - https://pubmed.ncbi.nlm.nih.gov/...
- Clinical Guidelines 2021 Rheumatoid Arthritis. www.cr.minzdrav.gov.ru/... Accessed March 18, 2022.
- Nasonov EL, Olyunin YuA, Lila AM. Rheumatoid arthritis: the problems of remission and therapy resistance. Nauchno-Prakticheskaya Revmatologiya = Rheumatology Science and Practice. 2018;56(3):363-271 (In Russ.) doi: 10.14412/1995-4484-2018-263-271
- Clinical Guidelines 2021 Rheumatoid Arthritis. www.cr.minzdrav.gov.ru/... Accessed March 18, 2022.
- Nasonov EL, Olyunin YuA, Lila AM. Rheumatoid arthritis: the problems of remission and therapy resistance. Nauchno-Prakticheskaya Revmatologiya = Rheumatology Science and Practice. 2018;56(3):363-271 (In Russ.) doi: 10.14412/1995-4484-2018-263-271
- Clinical Guidelines 2021 Rheumatoid Arthritis. www.cr.minzdrav.gov.ru/... Accessed March 18, 2022.
- Nasonov EL, Olyunin YuA, Lila AM. Rheumatoid arthritis: the problems of remission and therapy resistance. Nauchno-Prakticheskaya Revmatologiya = Rheumatology Science and Practice. 2018;56(3):363-271 (In Russ.) doi: 10.14412/1995-4484-2018-263-271
- Felson DT, Anderson JJ, Boers M, Bombardier C, Chernoff M, Fried B, et al. The American College of Rheumatology preliminary core set of disease activity measures for rheumatoid arthritis clinical trials. Arthritis Rheum. 1993;36(6):729–740. doi: 10.1002/art.1780360601
- Ramey DR, Raynauld JP, Fries JF. The health assessment questionnaire 1992: status and review. Arthritis Care Res. 1992;5(3):119– 129. doi: 10.1002/art.1790050303
- Anderson JK, Zimmerman L, Caplan L, Michaud K. Measures of rheumatoid arthritis disease activity. Arthritis Care Res (Hoboken). 2011;63 Suppl 11:S14–36. doi: 10.1002/acr.20621
- Cella D, Yount S, Sorensen M, Chartash E, Sengupta N, Grober J. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–819
- Electronic source ACR Response Criteria in Rheumatoid Arthritis Clinical Trials https://www.quanticate.com/.... Access date 01.03.2024
- Electronic source : https://clinicaltrials.gov DATED September 15, 2021 (NCT02760368; NCT03120949; NCT02760407; NCT02760433). Access date 19.02.2024.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Apr;81(4):469-479. doi: 10.1136/annrheumdis-2021-219876. Epub 2021 Aug 3.
- Electronic resource: https://clinicaltrials.gov/... Access date 19.02.2024.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Electronic resource: https://clinicaltrials.gov/... Access date 19.02.2024.
- Feist E, Fatenejad S, Grishin S, Korneva E, Luggen ME, Nasonov E, Samsonov M, Smolen JS, Fleischmann RM. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Dec;81(12):1661-1668. doi: 10.1136/ard-2022-222630. Epub 2022 Sep 15.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Apr;81(4):469-479. doi: 10.1136/annrheumdis-2021-219876. Epub 2021 Aug 3.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Feist E, Fatenejad S, Grishin S, Korneva E, Luggen ME, Nasonov E, Samsonov M, Smolen JS, Fleischmann RM. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Dec;81(12):1661-1668. doi: 10.1136/ard-2022-222630. Epub 2022 Sep 15.
- Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Apr;81(4):469-479. doi: 10.1136/annrheumdis-2021-219876. Epub 2021 Aug 3.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Feist E, Fatenejad S, Grishin S, Korneva E, Luggen ME, Nasonov E, Samsonov M, Smolen JS, Fleischmann RM. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Dec;81(12):1661-1668. doi: 10.1136/ard-2022-222630. Epub 2022 Sep 15.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Apr;81(4):469-479. doi: 10.1136/annrheumdis-2021-219876. Epub 2021 Aug 3.)
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
- Feist E, Fatenejad S, Grishin S, Korneva E, Luggen ME, Nasonov E, Samsonov M, Smolen JS, Fleischmann RM. Olokizumab, a monoclonal antibody against interleukin-6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by tumour necrosis factor inhibitor therapy: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Dec;81(12):1661-1668. doi: 10.1136/ard-2022-222630. Epub 2022 Sep 15.
- Instructions for medical use of the drug Artlegia, registration certificate LP- 006218 dated 21.05.2020, updated on 19.02.2024.
- Instructions for medical use of tocilizumab LSR-003012/09 and LP-003186. Electronic resource https://grls.rosminzdrav.ru/ date of access 26.01.2019
- Instructions for medical use of the medicinal product Kevzara, registration certificate No. LP-005185 dated 19.11.18
- Instructions for medical use of the drug Artlegia, registration certificate LP- 006218 dated 21.05.2020, updated on 19.02.2024.
- Instructions for medical use of tocilizumab LSR-003012/09 and LP-003186. Electronic resource https://grls.rosminzdrav.ru/ date of access 26.01.2019
- Instructions for medical use of the medicinal product Kevzara, registration certificate No. LP-005185 dated 19.11.18
- Instructions for medical use of the drug Artlegia, registration certificate LP- 006218 dated 21.05.2020, updated on 19.02.2024.
- Electronic resource: https://clinicaltrials.gov/.... Access date 19.02.2024.
- Nasonov E, Fatenejad S, Feist E, Ivanova M, Korneva E, Krechikova DG, Maslyanskiy AL, Samsonov M, Stoilov R, Zonova EV, Genovese M. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis. 2022 Apr;81(4):469-479. doi: 10.1136/annrheumdis-2021-219876. Epub 2021 Aug 3. (
- Electronic resource: https://clinicaltrials.gov/... . Access date 19.02.2024.
- Smolen JS, Feist E, Fatenejad S, Grishin SA, Korneva EV, Nasonov EL, Samsonov MY, Fleischmann RM; CREDO2 Group. Olokizumab versus Placebo or Adalimumab in Rheumatoid Arthritis. N Engl J Med. 2022 Aug 25;387(8):715-726. doi: 10.1056/NEJMoa2201302.
































